Commissioned Audit (Global)
Entrusted audit
The whole process of GMP compliance service is in place in one step.
1. Commissioned audit
Commissioned audit is also the third party audit of GMP. Combined with cGMP regulations and appendix requirements, as well as relevant national laws and regulations, the quality management system of pharmaceutical enterprises entrusted to be audited is systematically audited and evaluated, its authenticity, correctness, compliance, legality and traceability are reviewed, and GMP audit reports are issued. Enterprises rectify deficiencies and defects in R & D, production and operation according to the audit reports, ensure the stable and compliant operation of its quality management system.
Now more and more MAH enterprises choose third-party audit or commissioned audit, can help MAH enterprises because of the lack of personnel to carry out third-party audit. To solve the dilemma of enterprises, especially in the early stage of MAH, enterprises will face a large number of new raw materials or accessories suppliers of audit.

2. Scope of GMP third-party audits
Domestic and global third-party audit services, Durst currently helps a number of companies to conduct global supplier audits.
API manufacturers |
Preparation manufacturer |
Pharmaceutical packaging material manufacturers |
Contract manufacturers, external laboratories and service providers, etc. |
|
|
|
|
|
| According to the needs of customers, in accordance with the European and American GMP and NMPA requirements, the domestic and foreign factories to audit. | According to the customer's needs, conduct third-party inspection or due diligence on the preparation supplier. | Global audits of pharmaceutical packaging manufacturers in accordance with customer requirements.
|
Perform FAT or laboratory and service provider assessment or due diligence on equipment manufacturers according to customer needs. |
3. The service process of GMP third-party audit.
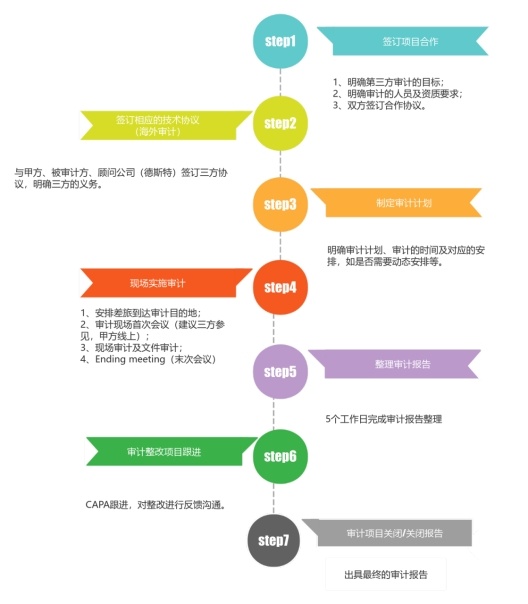
4. Durst Advantage:
1. Have a complete set of problems found, reply to official opinions, formulate rectification measures, guide the implementation of rectification measures, and follow up the effectiveness of the implementation of rectification measures to solve all problems found, and finally successfully pass the audit and official inspection service system;
2: professional team, a number of well-known foreign experts to join the industry, rich experience in auditing. Used to conduct GMP audits for a number of companies; Durst has mostly European and American audit experts with QP qualifications to help companies conduct QP audits;
3: Location advantage. Durst has offices in East China, South China and Southwest China, which is more convenient for face-to-face communication and investigation with enterprises..
4: one-stop, all-round technical services, etc.
5. Durst success story (part):
Durst helped a well-known enterprise in Zhuhai to complete the audit of raw materials in Denmark and the audit of raw materials in France;
Durst helped Ping An Yanye Yi MAH project of a well-known Zhejiang raw materials enterprise on-site audit.

|
Yangzijiang Pharmaceutical Group Nanjing Hailing Pharmaceutical Co., Ltd. |

|
Shenzhen Zhonghe Hyderway Biotechnology Co., Ltd. |
|
|
North Hunan Wellman Pharmaceutical Co., Ltd. |

|
Shanxi Nuocheng Pharmaceutical Co., Ltd. |

|
Shenzhen Haibin Pharmaceutical Co., Ltd |
|
|
Anshi Pharmaceutical (Zhongshan) Co., Ltd. |

|
Zhongshan Wanhan Pharmaceutical Co., Ltd. |

|
Unnamed Biological Group |

|
Zhejiang Jingwei Pharmaceutical Co., Ltd. ...... |

