QP Conformance Declaration
1. QP Conformance Statement (QP Declaration)
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas.
According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
PIC/S member countries or regions, involving (currently increasing):
Argentina, Australia, Austria, Belgium, Canada, Chinese Taipei, Croatia, Cyprus, Czechoslovakia, Denmark, Estonia, Finland, France (2), Germany (2), Greece, Hong Kong, Hungary, Iceland, Indonesia, Ireland, Israel, Italy, Japan, South Korea, Latvia, Liechtenstein, Lithuania, Malaysia, Malaysia, Netherlands, New Zealand, Norway, Poland, Portugal, Romania, singapore, Slovakia, Slovenia, South Africa, Spain, Sweden, Switzerland, Ukraine, United Kingdom (2), United States, etc.
Classification of 2. QP Conformance Declaration (Classification of the QP Declaration)
1, divided into API compliance statement and FDF compliance statement:
(1) API-qualified QP can issue API's GMP compliance declaration;
(2) QP with preparation qualification can issue GMP compliance declaration of preparation.
3. DST's QP Conformance Declaration Advantage (QP Declaration of DST stated advantage)
DST's QP experts are QP qualified for both API and formulation. If the enterprise is involved in the stock solution and preparation, it can help the enterprise to complete the inspection of the stock solution and preparation at the same time, and issue the QP declaration certificate of API and preparation;
QP has the inspection and counseling experience of vaccines, biological products, chemical drugs and API enterprises, and helps enterprises to solve some practical GMP-related difficult problems in the inspection, so as to successfully pass the formal international certification in the later period;
DST's QP has rich working experience in China, understands many corporate cultures and inspection methods in China, and can better understand some working methods of enterprises during the implementation process;
When DST performs QP compliance inspection, it will simultaneously arrange experts to guide the inspection on site so that the inspection can be carried out smoothly, and at the same time, it will help the enterprise to pass the inspection smoothly;
DST experts will help enterprises to rectify defects in QP compliance inspection, help enterprises to provide GMP level and obtain QP compliance statement smoothly.
QP Declaration of Conformance Case 4. DST (QP statement of DST)
Involving enterprises, including but not limited to: Liaoning Yisheng Biology, Beijing Zhaoyan Biology, Yaoming Biology, Ruike Biology, Hangzhou Aoya Biology, Shenzhen Kangtai Biology, Guangdong Raffles, Chinese Medicine Zhongsheng Biology, Beijing Kangle Health, Suzhou Prui Biology, Shanghai Borice Biology, Zhejiang Hengyu Biology, Jiangsu Zhixiang Biology, Guangzhou Anji Sheng Biology, etc.
Durst has rich practical experience in helping enterprises successfully pass the QP compliance declaration, and can quickly help enterprises pass the inspection smoothly. Durst has experience in remote and on-site QP compliance declaration inspection.

|

|
QP Audit Image |
QP Audit Image |
五、DST的QP审计前期准备(QP audit preliminary preparation for DST)
Legal Basis
The main regulatory basis for the QP Declaration of Conformance is the EU GMP Guidelines, including the text and appendices.
Overall process
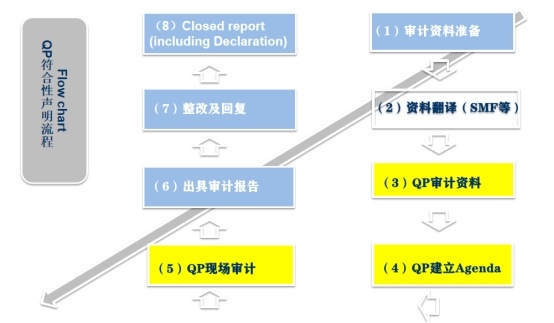
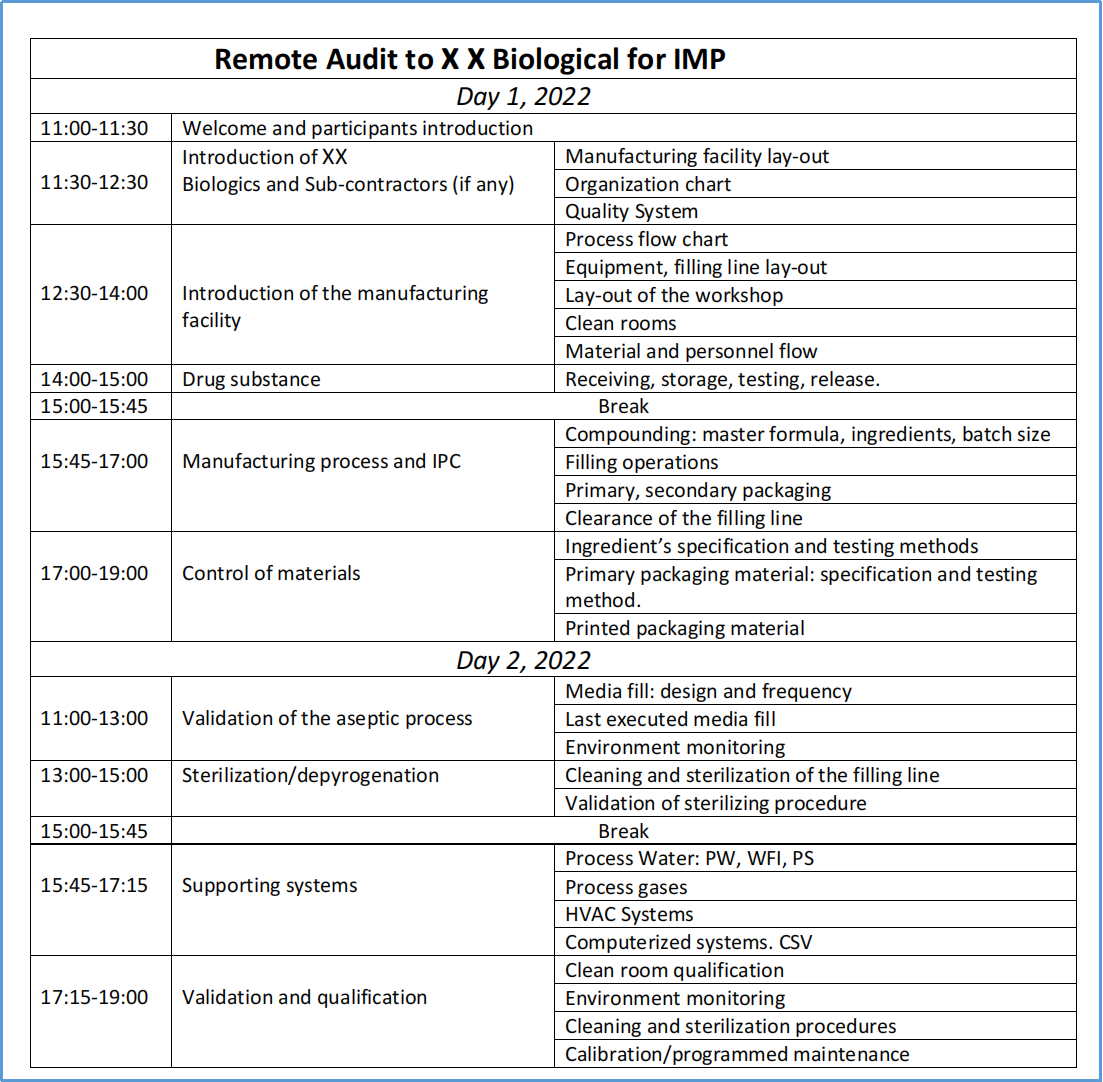
3. Inspection schedule
The QP Declaration Statement process and matters for the DST 6.

