CSV Computerized System Validation
Release time:
2024-10-25
1. Computer system verification (CSV verification) is an important part of quality assurance in the pharmaceutical and related industries, and it is the part that must be verified in the 2010 GMP appendix. Durst's validation team has a deep background in the pharmaceutical industry, validation consulting, and a deep understanding of domestic and international GMP regulations and rich practical experience. Can provide you with computer verification services for compliance with GMP requirements such as FDA, EU, TGA, NMPA, etc.
1. Computer system verification (CSV verification)It is an important part of quality assurance in the pharmaceutical and related industries, and it is the part that must be verified in the 2010 GMP appendix. Durst's validation team has a deep background in the pharmaceutical industry, validation consulting, and a deep understanding of domestic and international GMP regulations and rich practical experience. Can provide you with computer verification services for compliance with GMP requirements such as FDA, EU, TGA, NMPA, etc.
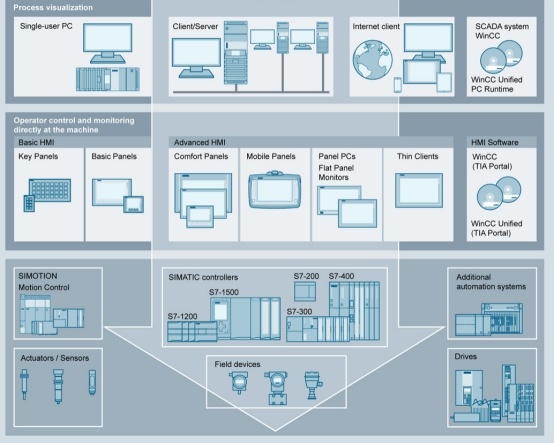
2. Verification scope:
Durst Computer Systems Verification TeamHe has provided verification services for many foreign-funded, state-owned enterprises and other pharmaceutical companies and software developers in China. He is very familiar with computer system verification and has GAMP 5 verification capability. The verification scope includes but is not limited:
(1) System software:
ERP enterprise management system, WMS warehouse management system, WCS warehouse control system (Lizhu Group, Shenzhen Zhonghe Hedway, Shanxi Zhendong Pharmaceutical, Shanghai Danrui Pharmaceutical, Shenzhen Jiuxin Pharmaceutical, Zhuhai Fitelan, etc.)
QMS Quality Management System (Trackwise \Shanghai Hongyi)
LIMS Laboratory Management System (Oz Pharmaceutical Project)
DMS File Management System (Shanghai Hongyi)
(2) Production equipment and public system
BMS, EMS system (Respril Project, Centone Pharmaceuticals, Rizumab, etc.)
PMS environmental monitoring system (Xiantong Pharmaceutical, Lizuzumab, etc.)
HMI/PLC man-machine operation interface/programmable logic controller (stock solution equipment, purification equipment, preparation equipment, such as bottle washing machine, tunnel oven, filling machine, tablet press, coating machine, etc.) (Shengnuo Biology, Xiantong Pharmaceutical, Lizuzumab, etc.)
PCS production control system (Shengnuo Bio, Xiantong Pharmaceutical, Lizuzumab, etc.)
DCS distributed control system (Shengnuo Bio, Xiantong Pharmaceutical, Lizuzumab, etc.)
SCADA DCS, etc. (Shengnuo Bio, Xiantong Pharmaceutical, Lizuzumab, etc.)
(3)QC instruments and equipment:
ELN CDS (Shimadzu/Agilent/Waters Online Edition Software), stand-alone instruments (UV, IR, PCR instruments, fluorescence instruments, cell counters, etc.) (North China Pharmaceutical, Shanxi Nuocheng Pharmaceutical, North Hunan Wellman, Shenzhen Zhonghe Haide Wei, Guangdong and Macao Pharmaceutical, Jiangsu Keben Pharmaceutical, Guangdong Xinghao Pharmaceutical, etc.)
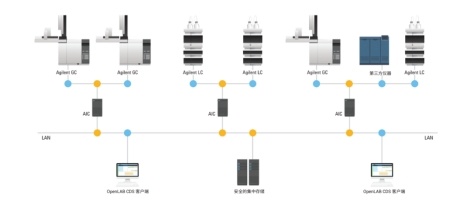
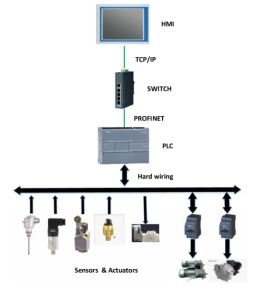
3. Verification process:
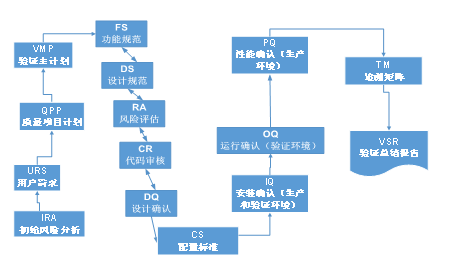
3.1V Validation Model
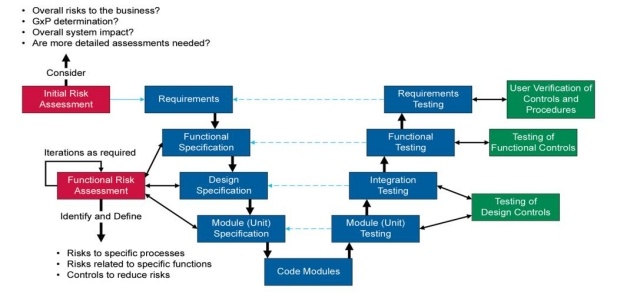
3.2 Validation Control Point
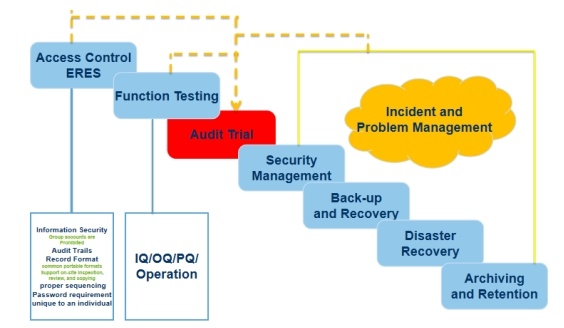
4. Validation process and delivery
5 Specific cases
ERP-WMS System Verification
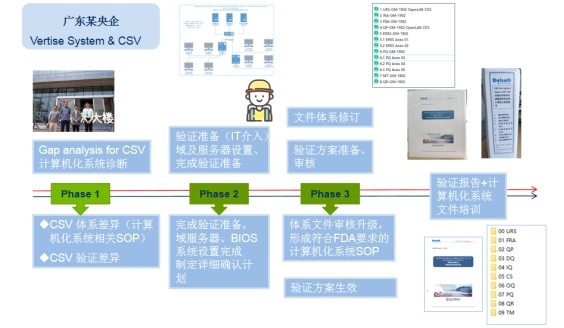
|
|
Durst advantage: DurstHave a professional team according to the function and actual use of the customer's computerized system, make a list of computerized systems, and classify them, carry out verification services according to the current standards, and help the customer's project meet the FDA/EMA/PICS/WHO verification requirements. |
Durst Success Story (partial):

|
North China Pharmaceutical: CSV verification of laboratory instruments and equipment, involving data integrity and computerized system verification training. |

|
Zhejiang Jingwei Pharmaceutical Co., Ltd. (FDA cGMP project): Laboratory data integrity and CSV training and on-site guidance to help companies successfully pass FDA cGMP certification inspections. |

|
Xiangbei Wellman Pharmaceutical Co., Ltd. (NMPA and European and American GMP standards): Validation of the laboratory online version of the chromatographic system and the stand-alone version of the instrument. |

|
Shenzhen CNNC Hyde Bio (EU and PIC/S standards): 1. Overall solution of laboratory data backup and verification service; 2. Computerized system verification of laboratory instruments and equipment. |
|
|
Shanxi Nuocheng Pharmaceutical Co., Ltd. (PIC/S standard): Overall PIC/S project, CSV project involving key production equipment; and CSV project for laboratory instruments; Data Integrity Guidance. |
|
|
Anshi Pharmaceutical (Zhongshan) Co., Ltd. (FDA cGMP and PIC/S project) Data integrity guidance and production data integrity training, CSV system construction, personnel training, etc. |
|
|
Jiangsu KeBen Pharmaceutical Co., Ltd. Computerized System Verification Project (CSV) Involving QC laboratory HPLC, GC, LC, FTIR, UV, etc. Production System Validation |
|
|
Zhuhai Lizhu Group (FDA cGMP and NMPA): involving Lizhu microspheres, Lizhu monoclonal antibody and Lizhu pharmaceutical factory. 1. Lenovo cloud CSV project, laboratory CSV training, data integrity guidance review, CSV system construction, etc. 2. WMS System Verification Project |

|
Beijing Lianxin Pharmaceutical (NMPA Standard) Drug Traceability System Verification Project, GAMP Category 5 Customized Software Test software code, verify code report analysis, and verify overall execution. |

|
Shanghai Dan Rui Biomedical Technology (NMPA and FDA cGMP) Infor ERP & production control system, involving the design review of the system, the overall verification of the system on-line, PQ verification after delivery, etc. |
|
|
Kangfang Biomedical Co., Ltd. (FDA cGMP and NMPA) CSV items of production equipment |

|
Zhangjiagang Huanyu Pharmaceutical Equipment Co., Ltd. (FDA cGMP and NMPA) Overall validation guidance for key production equipment and CSV validation overall project services. |

|
Guangdong Xinghao Pharmaceutical (FDA cGMP) CSV verification of key equipment and instruments involved in the laboratory, CSV verification training, etc., and zero defect inspection in CSV. |

|
Shanghai Hongyi Software Technology Co., Ltd. (DMS Project) Software development guidance and software CSV verification guidance. |

|
Guangzhou Zhuoge Information Technology Co., Ltd. (software development) ...... |
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

