QP Conformance Declaration
Release time:
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
1. QP Conformance Statement (QP Declaration)
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas.
According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
PIC/S member countries or regions, involving (currently increasing):
Argentina, Australia, Austria, Belgium, Canada, Chinese Taipei, Croatia, Cyprus, Czechoslovakia, Denmark, Estonia, Finland, France (2), Germany (2), Greece, Hong Kong, Hungary, Iceland, Indonesia, Ireland, Israel, Italy, Japan, South Korea, Latvia, Liechtenstein, Lithuania, Malaysia, Malaysia, Netherlands, New Zealand, Norway, Poland, Portugal, Romania, singapore, Slovakia, Slovenia, South Africa, Spain, Sweden, Switzerland, Ukraine, United Kingdom (2), United States, etc.
Classification of 2. QP Conformance Declaration (Classification of the QP Declaration)
1, divided into API compliance statement and FDF compliance statement:
(1) API-qualified QP can issue API's GMP compliance declaration;
(2) QP with preparation qualification can issue GMP compliance declaration of preparation.
3. DST's QP Conformance Declaration Advantage (QP Declaration of DST stated advantage)
DST's QP experts are QP qualified for both API and formulation. If the enterprise is involved in the stock solution and preparation, it can help the enterprise to complete the inspection of the stock solution and preparation at the same time, and issue the QP declaration certificate of API and preparation;
QP has the inspection and counseling experience of vaccines, biological products, chemical drugs and API enterprises, and helps enterprises to solve some practical GMP-related difficult problems in the inspection, so as to successfully pass the formal international certification in the later period;
DST's QP has rich working experience in China, understands many corporate cultures and inspection methods in China, and can better understand some working methods of enterprises during the implementation process;
When DST performs QP compliance inspection, it will simultaneously arrange experts to guide the inspection on site so that the inspection can be carried out smoothly, and at the same time, it will help the enterprise to pass the inspection smoothly;
DST experts will help enterprises to rectify defects in QP compliance inspection, help enterprises to provide GMP level and obtain QP compliance statement smoothly.
QP Declaration of Conformance Case 4. DST (QP statement of DST)
Involving enterprises, including but not limited to: Liaoning Yisheng Biology, Beijing Zhaoyan Biology, Yaoming Biology, Ruike Biology, Hangzhou Aoya Biology, Shenzhen Kangtai Biology, Guangdong Raffles, Chinese Medicine Zhongsheng Biology, Beijing Kangle Health, Suzhou Prui Biology, Shanghai Borice Biology, Zhejiang Hengyu Biology, Jiangsu Zhixiang Biology, Guangzhou Anji Sheng Biology, etc.
Durst has rich practical experience in helping enterprises successfully pass the QP compliance declaration, and can quickly help enterprises pass the inspection smoothly. Durst has experience in remote and on-site QP compliance declaration inspection.
 |  |
QP Audit Image | QP Audit Image |
5.QP audit preliminary preparation for DST
Legal Basis
The main regulatory basis for the QP Declaration of Conformance is the EU GMP Guidelines, including the text and appendices.
Overall process
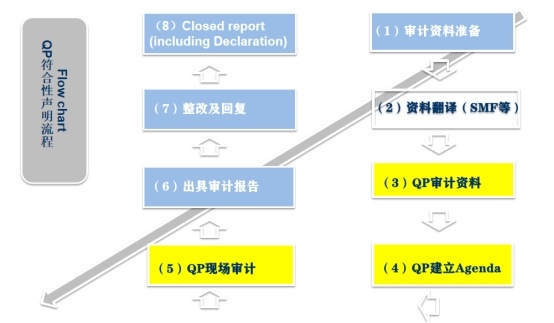
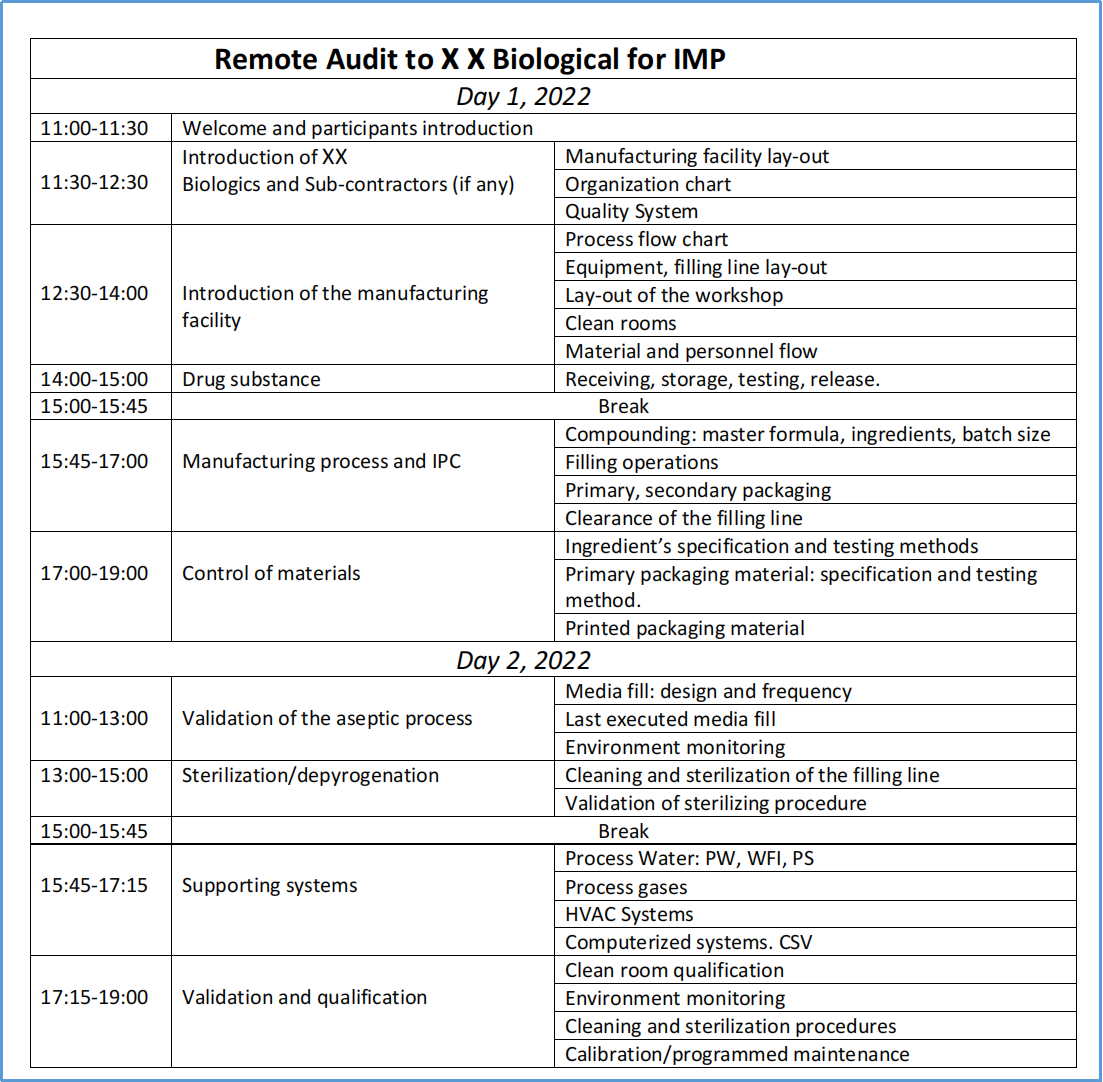
3. Inspection schedule
The QP Declaration Statement process and matters for the DST 6.
Previous
Previous:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

