CDE: Notice on Trial Submission of Electronic Application Data for Drug Registration by Network Transmission
Release time:
2024-07-05
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
In recent years, since joining ICH in 2017, NMPA has been vigorously promoting the construction of an electronic platform for drug declaration. In order to improve the application service level of "Internet drug supervision", CDE has established a network transmission channel for electronic declaration materials. On the basis of using CD to submit electronic declaration materials, CDE has increased the network transmission mode, providing various choices for applicants to submit electronic declaration materials, so as to improve the submission efficiency of electronic declaration materials of applicants. The following is the relevant content of the announcement:
ICH guidelines are technical guidelines for drug development, including four categories, namely quality (Quality,Q), safety (Safety,S), effectiveness (Efficacy,E) and multidisciplinary (Multidisciplinary,M). In order to unify the format and content requirements of drug application materials, ICH has set up M4(CTD) and M8(eCTD) topics in the multidisciplinary classification under the framework of the overall technical guidance system.

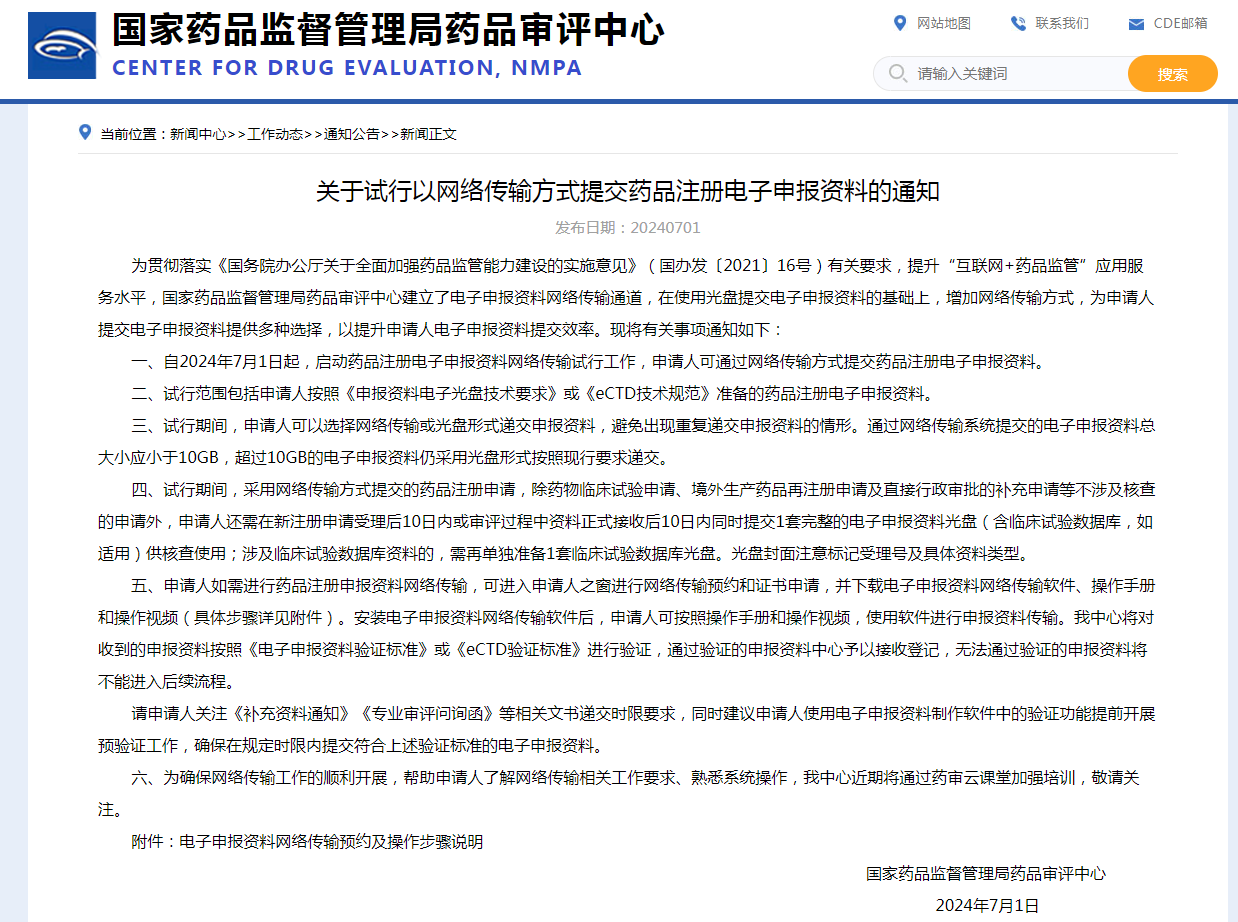
CTD is an internationally recognized format for compiling drug application materials, which is divided into five modules: module one, administrative documents and drug information; Module two, general technical document summary; Module three, quality; Module four, non-clinical trial report; Module five, clinical research report. Among them, module one is a regional requirement, the specific content and format are stipulated by the corresponding regulatory agencies, and module 2. 3. four and five are international general requirements.
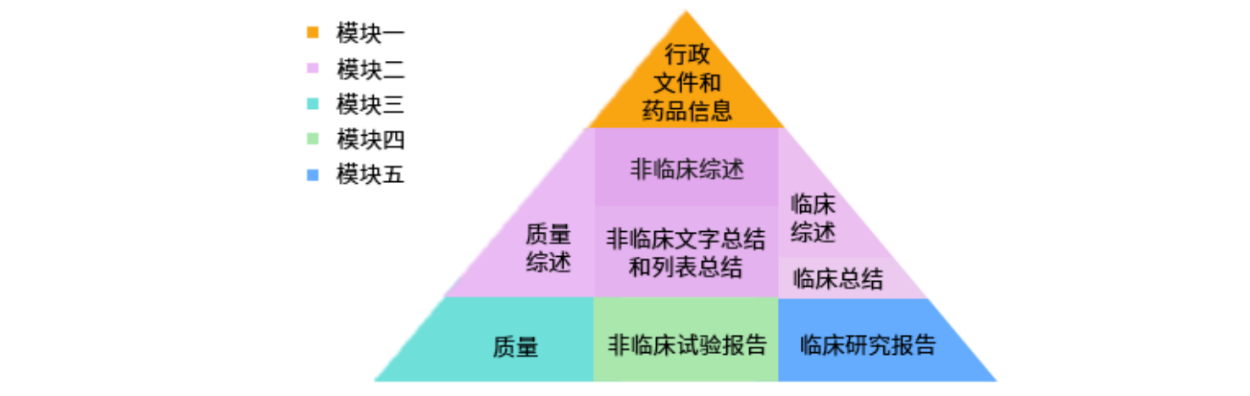
Electronic Common Technical Document (eCTD) is an electronic presentation and management method for Common Technical Document (CTD), which organizes documents based on CTD file structure and eCTD technical specifications through Extensible Markup Language (XML) technology and is used as a registration document technical format for drug registration declaration and review. eCTD makes the preparation, declaration, acceptance, review, life cycle management and file storage of the application data more convenient and economical, which not only ensures the quality of the data declaration, but also improves the efficiency of the review, and more importantly, maintains the global universality and consistency of the format, content and standard requirements of the application data.
ICH issued eCTD Technical Specification V3.2.2 in 2008, and eCTD technical specifications are widely used in many countries and regions. Since May 30, 2017, CDE has issued a notice on soliciting opinions on the "Structure of Electronic General Technical Documents for Drugs (Draft for Comments)" and the "Guidelines for the Application of Electronic General Technical Documents for Chemical Generic Drugs (Draft for Comments)". NMPA and CDE have issued ten relevant notices, of which NMPA issued a notice on September 30, 2021 on the implementation of the application of electronic general technical documents for drugs (No. 119, 2021), it is clearly pointed out that starting from December 29, 2021, applications for listing licenses for chemical drug registration classifications 1 and 5.1, therapeutic biological products 1 and preventive biological products 1 can be declared according to eCTD; On January 29, 2023, CDE's notice on public solicitation of opinions on "eCTD Implementation Guidelines V1.1 (Draft for Comments)" and "eCTD Verification Standard V1.1 (Draft for Comments), A single PDF file should be controlled within 500MB and modified to within 200MB.
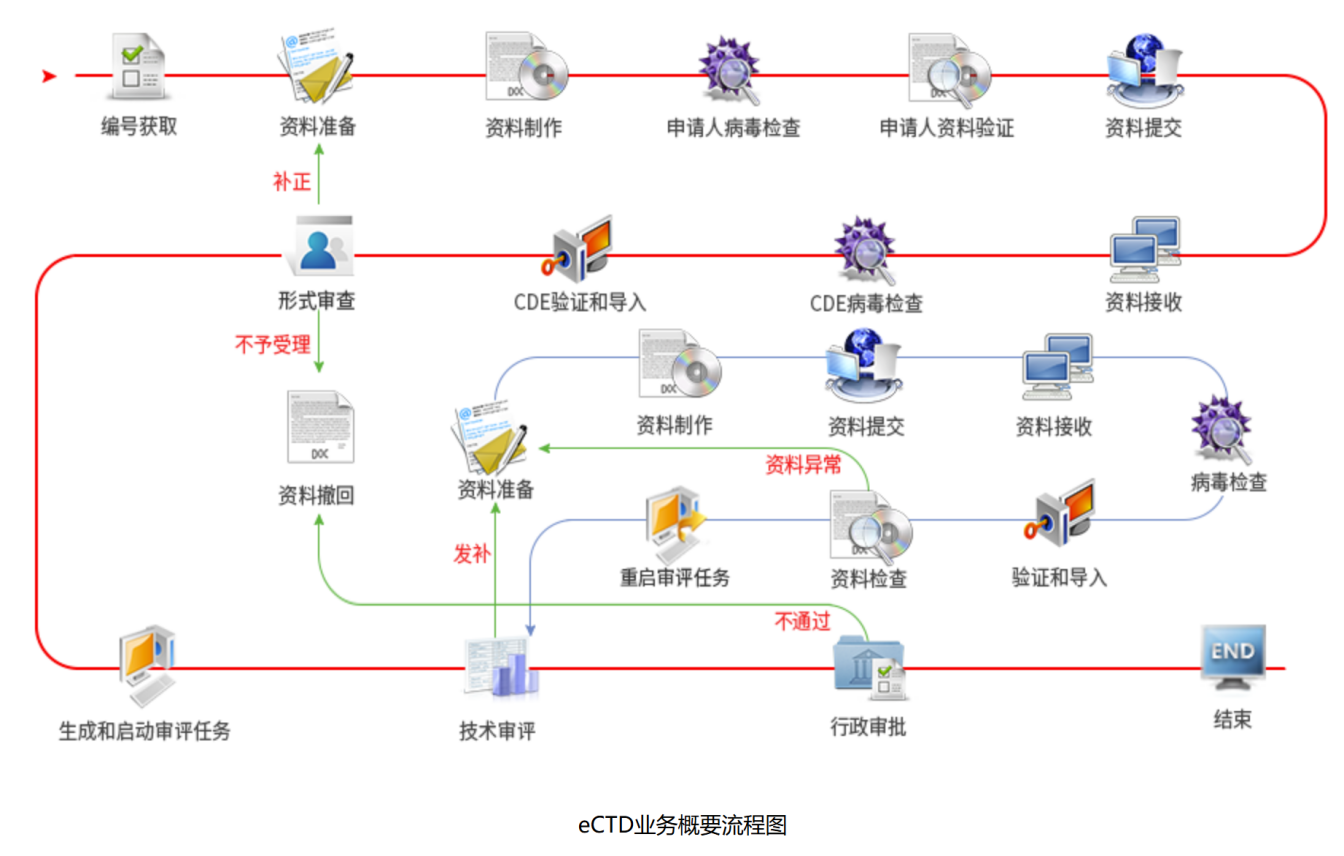
The following is a description of the eCTD business profile process:
Key words:
Previous
Related Blog
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Argument! Hengrui Warning Letter, Is It FDA's Political Sanction?
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
2024-10-25
Durst DST continues to pay attention to the drug regulatory policies of countries around the world to help companies go to sea, and the following is the drug regulatory situation in Southeast Asian countries and regions.
2024-10-25

