Argument! Hengrui Warning Letter, Is It FDA's Political Sanction?
Release time:
2024-10-25
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
The positive view, which is influenced by political factors, has three reasons:
Part 1: Politics. Based on political theory, first of all, considering that Hengrui has a market value of 300 billion yuan, it is indeed a very high market value, and it is a leading pharmaceutical company in China. The company is strong and is targeted by the U.S. government. The U.S. FDA is influenced by the U.S. political group and must deal with this Chinese pharmaceutical brother. The reason is clear and clear, and everyone can see it.
Part 2: Conspiracy theory. Basis: Hengrui's inspection was reported as FDA's "flight inspection", which means FDA had an idea for a long time, or, in a bad word, a "premeditated" inspection for a long time. Before I came, I thought about it and "set up" this enterprise. Therefore, it seems very reasonable to try to trick you.
Three: Threat theory. Strong R & D strength involves R & D, which is the field that Americans least want to see. Americans want Chinese to make low-end products, raw materials, generic drugs, etc. Therefore, it is equivalent to robbing Americans of their jobs and must intervene! Must be dealt! Moreover, the US FDA is under the intervention of the US government. Americans pull out the hidden dangers early, the promoter here is the top pharmaceutical companies or Jewish capital in the United States, the case is similar to the sanctions against Huawei.
The opposing side argues that it is not all political, for the following reasons:
Americans understand politics and whether the FDA can be easily controlled by the government. The author believes that this is indeed possible. However, the author believes that the US government's ability to control the FDA is limited. In response to this problem, the author has discussed this issue with many FDA inspectors. Of course, the inspectors may not tell the truth. The US government and the FDA are truly products of commerce. For the US government, it is necessary for these technicians to do administrative work, but it is not so easy. Because many "civil servants" in FDA are relatively loose, because there are almost 20,000 people in FDA in the United States, it is hard to say who will kill them. The FDA inspector said that the FDA would let some companies go, but did not say which company the FDA had to kill.
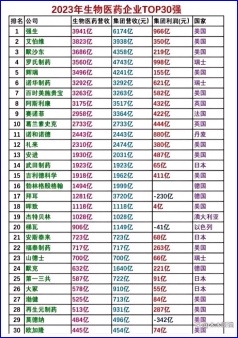
If the U.S. government really wants sanctions, it can use the way it used to deal with drugs and organisms, directly put them on the entity list, and kill them with one shot, without making a detour. But compared with other industries, the pharmaceutical industry has its particularity, and, looking at Hengrui's products, what is the actual sales? Let's look at the data of Hengrui in 2023, 22.82 billion yuan, profit 4.302 billion yuan, and then look at the following table (Table 1). Hengrui's current sales may not be as good as that of a large pharmaceutical company in the United States.
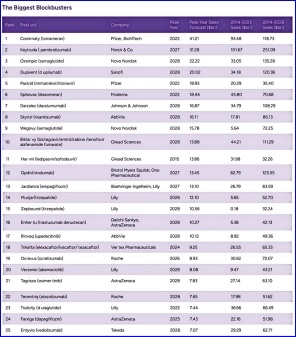
Of the 487 drugs approved by the FDA from 2014 to 2023, 17 belong to the first echelon, with annual sales exceeding $10 billion; 42 drugs with more than $5 billion; and the other 114 drugs will sell for more than $2 billion a year. These products are $100 in sales and are all single items. (Table 2) Therefore, in terms of strength, Hengrui still has a huge gap, and its annual sales cannot even reach the taillights of top foreign companies. At least our first brother still has a long way to go.
Let's take a look at Hengrui's sea data. After reading it, you will probably believe that Hengrui will not receive attention in the US Customs. From 2019 to 2022, Hengrui's overseas market revenue was 0.632 billion yuan, 0.758 billion yuan, 0.617 billion yuan and 0.779 billion yuan respectively. In 2023, the overseas market achieved revenue of 0.617 billion yuan. Compared with 2022, Hengrui's overseas market revenue also decreased by 20%. This profit is estimated to be less than a fraction of the money made by any large foreign pharmaceutical in China.
Table 1 |
Table 2 |
|
|
Kill the monkey and respect the chicken. Did the FDA do this on purpose? The counterside believes that this possibility exists. But it doesn't necessarily follow that the FDA has been doing something interesting. Just like "kill monkey respect chicken". This is indeed stated in the FDA's warning letter of reason and intention. The agency of the FDA is huge, but for so many countries around the world, so many pharmaceutical companies, so many product lines, they lack enough energy to manage. So, the FDA likes to challenge the powerful and likes to kill the faucet. As a typical let everyone wake up, in order to strengthen management. In other words, brothers, I can kill your boss, not to mention you "scum". Moreover, it is quite easy for people who retire from the FDA to come out and find a job.
In fact, when you check the FDA's warning letter, you will often see the list of some large enterprises such as Pfizer and Novartis. As many pharmaceutical companies in China have entered the US market, it is time to kill a monkey. So, this opportunity is absolutely a godsend for the FDA. It's a pity that Hengrui is not lucky.
As a matter of fact, there is nothing wrong with giving a warning letter to tell the truth about these Findings found in Hengrui 483. It's just that Brother Pharma is really careless. For example, the problem of data integrity letter: the production manager prints multiple batches of records in advance and changes the records at will. See this, ladies and gentlemen. Do you think the FDA's political consciousness is so high? I don't think so. Tieguai Liu believes that it will be "embarrassing" to put these problems on the surface of any official inspection in time ".
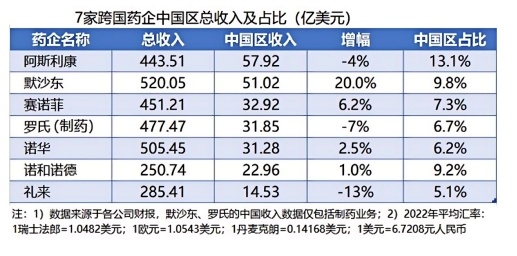
In terms of politics, the economic accounts are calculated. Who is beneficial to the mutual sanctions between the Chinese and American pharmaceutical industries? Please look at the table below. Tieguai Liu only took out the data for 2022 (Table 3) and showed it to everyone. I think in terms of numbers, American Jews know how to choose with their eyes closed. Just like the U.S. government put Yao Ming on the entity list and found that the account was wrong, because if China imposed reciprocal sanctions on these pharmaceutical companies, I really don't know who was unlucky. The old beauty never suffers from making money.
For heavy-duty enterprises such as Huawei and Dajiang, their income does not need to be turned away from Liu. They have indeed become what Americans think of as their enemies and have really robbed them of their jobs. And, even with reciprocal sanctions, China cannot take advantage. Because Apple was mainly produced in China before, don't you want your GDP?
Table 3
Do the products developed by Hengrui have "banknote ability"? In fact, the products of Hengrui are mainly traditional chemical anti-tumor drugs, although these products have good profits. However, there are many alternative anti-tumor products. Not those big Mac products that biopharmaceuticals can soar. In addition, Hengrui's R & D investment is still far behind that of world-class European and American companies.
Finally, the author believes that the FDA has indeed played a role in "killing monkeys and respecting chickens" this time. Of course, Hengrui has put a lot of energy into research and development, ignoring the management risks of GMP, and there are real problems. Durst DST did a public interest event with Johns Hopkins University for the New Crown Project during 2022. At that time, I was lucky enough to have some private exchanges with their dean (Remark: the school has won more than 40 Nobel Prizes in medicine.) She believes that the FDA is still relatively weak under the influence of the government, which is also based on the particularity of medicine. On the contrary, just like Huahai, FDA can even give warning letters and send people to help enterprises improve quality and continue to supply to patients to avoid the risk of insufficient medication for patients.
Key words:
Related Blog
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Argument! Hengrui Warning Letter, Is It FDA's Political Sanction?
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
2024-10-25
Durst DST continues to pay attention to the drug regulatory policies of countries around the world to help companies go to sea, and the following is the drug regulatory situation in Southeast Asian countries and regions.
2024-10-25

