What happens when the root cause of the deviation is human error?
Release time:
2024-10-25
As technology advances, human error in manufacturing is becoming more apparent every day. In the pharmaceutical industry, more than 80% of deviations are caused by human error, but unlike process or equipment failures, human error is usually not investigated in depth, and despite the underlying cause, companies will eventually only provide retraining for the relevant employees, and this simple method is usually evaluated as "imperfect quality system". To this end, Amy and everyone together to see how to identify the root causes of human error and really reduce human error.
As technology advances, human error in manufacturing is becoming more apparent every day. In the pharmaceutical industry, more than 80% of deviations are caused by human error, but unlike process or equipment failures, human error is usually not investigated in depth, and despite the underlying cause, companies will eventually only provide retraining for the relevant employees, and this simple method is usually evaluated as "imperfect quality system". To this end, Amy and everyone together to see how to identify the root causes of human error and really reduce human error.
Why do people make human errors?
There are three main factors that are potential triggers for human error, namely task complexity, behavioral characteristics, and error-prone situations. In addition, there are a variety of precursors to human error that can cause errors to occur:
(1) Mission requirements
Time pressure (rush)
High workload (to have enough memory)
Perform multiple tasks simultaneously
Repetitive and monotonous work
Incorrect understanding
personal ability
Not familiar with the task.
lack of knowledge/proficiency/experience
Lack of effective communication
Insufficient problem-solving skills
Insufficient focus on key tasks
Illness and/or fatigue
Working environment:
Distraction and Interference
Change or deviation from regular
Noisy work environment
Cannot accept work culture
Humanity
Stress (limiting attention)
Habit mode
Assumptions (inaccurate mental picture)
complacency and overconfidence
Mentality
Inaccurate risk perception
Memory is limited
Human error investigation
After an error occurs, the risk impact of the error varies. It is best to decide whether to conduct a comprehensive human error investigation while considering the risk impact. The most commonly used method of investigation is fishbone diagram:
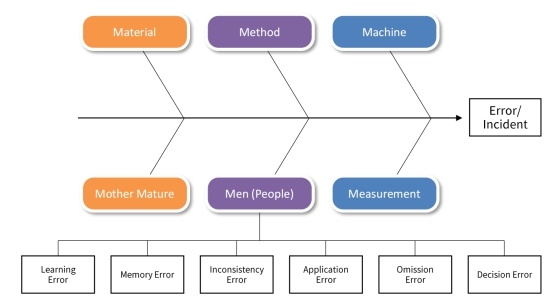
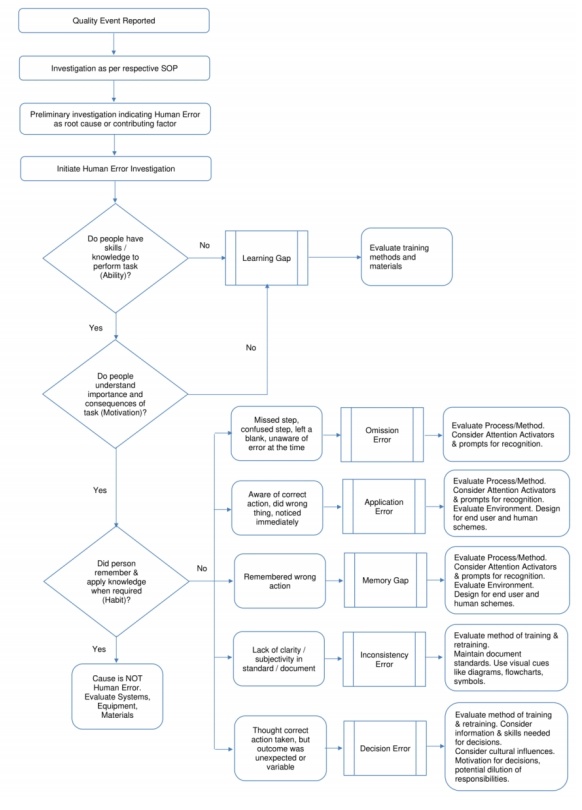
If the preliminary investigation indicates that the cause of the error was human error, the following investigation path will help further the investigation:
(1) Quality incident report;
(2) survey according to their respective sops
(3) Preliminary investigation indicates that human error is the root cause or contributing factor
(4) Initiate an investigation of human error (see the following flowchart for the investigation) and focus on the following points:
Do people have the ability and knowledge to do this?
Is the person aware of the importance of the task and the end result of the task?
Do people remember & apply knowledge (habits) when needed?
On the basis of the preliminary investigation, the real cause of human error should be further understood through the following methods:
(1) Investigators observe the operations of the analyst/operator
(2) Investigators interact with analysts/operators to understand the quality and gaps in processes and procedures.
(3) Get feedback from other operators on the task/possible cause
(4) Structured brainstorming
(5) If it is found that the cause of the error is related to the ability of personnel, it should be classified accordingly
(6) For system-related defects, the following assessment needs to be followed to understand whether the error is caused by one or more of the following factors:
Missing or inadequate processes (are the instructions in the procedure completely clear?)
Is the training adequate (Is the training adequate?)
Lack of experience (Is the person involved experienced?)
Communication Gaps (Is Oversight Adequate?)
Deliberate mistakes (are there any signs of unwisdom?)
Timely provision of required resources (is the infrastructure available to support the mission adequate?)
Stress conditions (physical and/or psychological) (Will fatigue play a role in this failure?)
Key words:
Related Blog
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Argument! Hengrui Warning Letter, Is It FDA's Political Sanction?
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
2024-10-25
Durst DST continues to pay attention to the drug regulatory policies of countries around the world to help companies go to sea, and the following is the drug regulatory situation in Southeast Asian countries and regions.
2024-10-25

