Supplier audit, colleagues need not worry, have reference
Release time:
2024-10-25
Supplier Management As part of the daily work of QA, materials are subject to strict qualification and on-site review before they are identified as qualified suppliers. As required by China GMP(2010 Edition), Chapter 10 (Quality Control and Quality Assurance), Section 7 (Evaluation and Approval of Suppliers) and Appendix (Computerized Systems):
Supplier Management As part of the daily work of QA, materials are subject to strict qualification and on-site review before they are identified as qualified suppliers. As required by China GMP(2010 Edition), Chapter 10 (Quality Control and Quality Assurance), Section 7 (Evaluation and Approval of Suppliers) and Appendix (Computerized Systems):
Article 255 stipulates: "The quality management department, in conjunction with relevant departments, conducts on-site quality audits of the quality systems of major material suppliers (especially manufacturers) and exercises veto power over suppliers whose quality assessments do not meet the requirements".
Article 257 stipulates: "The quality management department shall designate a person to be responsible for the quality assessment and on-site quality audit of material suppliers, and distribute the approved names of qualified suppliers".
Article 263 stipulates: "The quality management department shall sign a quality agreement with the main material supplier, in which the quality responsibilities of both parties shall be clearly defined".
Article 264 stipulates: "The quality management department shall conduct regular or on-site quality audits of material suppliers and review and analyze the results of material quality inspections, quality complaints and non-conformity handling records".
Article 12 (Appendix to Computerized Systems) states: "Enterprises shall, on the basis of the results of risk assessment, conduct hierarchical management of the software used (e. g. audits of software suppliers), evaluate the supplier's quality assurance system, and ensure that the software meets the needs of the enterprise".
The APIC (Active Pharmaceutical Ingredients Committee) updated the API Manufacturer Supplier Management Best Practice Guide (Second Edition) on March 18, 2024, which aims to establish a supplier management framework that not only meets official requirements, but also covers other supplier management related aspects that play a role in real life. "Supplier" means both material suppliers (including: raw materials, intermediates, reagents, solvents, packaging materials, processing aids) and service suppliers (e. g. contract laboratories, contract manufacturing, transportation, warehousing, calibration or critical IT services).
The full text is divided into nine parts, mainly from the supplier management, risk management, audit and quality agreement.
(I) supplier management
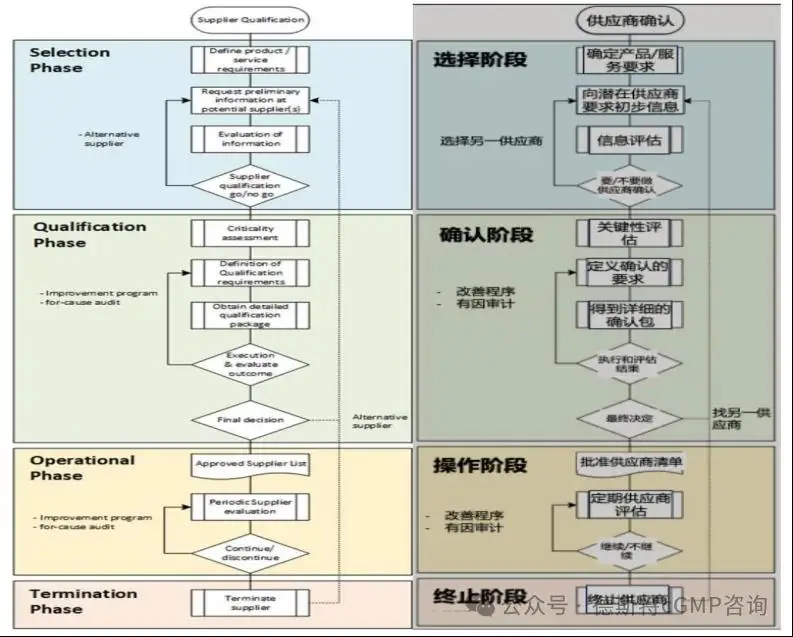
In the supplier management lifecycle, four phases can be defined:
Ø Selection stage: The purchasing department is usually the department responsible for providing (listing) potential suppliers;
Ø Qualification stage:
The qualification phase should basically cover two aspects:
-Preliminary evaluation of materials or services to assess the quality of the materials or services provided and their impact on the quality of the API;
-Evaluate the supplier's facilities and/or quality management system to assess whether the supplier can consistently provide materials or services of the required quality.
Both aspects need to be considered in parallel at the qualification stage.
Ø Operation phase:
Supplier performance evaluation includes:
-Quality aspects (e.g. material or service quality, audit results, compliance issues, deviation response, RFT %...)
-Other performance aspects (e.g. supply chain issues, on-time delivery).
The following table gives an example of the frequency of reassessments based on the overall supplier risk rating:
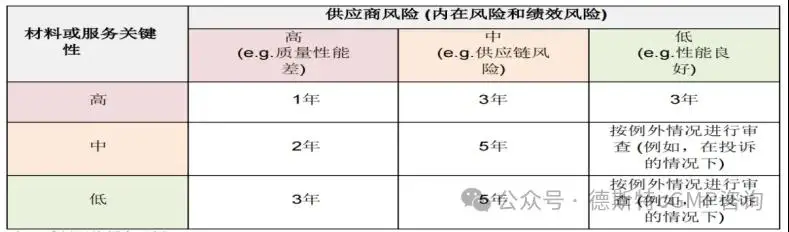
Ø Termination Phase
The valid qualification status of a supplier/service provider may be terminated or not reassigned for several reasons, such:
-The supplier has ceased to produce materials/provide services;
-the API manufacturer has discontinued the use of materials, suppliers or services;
-The supplier has serious problems (e. g. quality related problems without appropriate CAPA measures, negative audit output, supply chain problems, violation of agreed quality requirements).
If disqualified due to quality issues, the potential impact on all relevant materials in stock, in transit, or shipped to the customer should be assessed. This assessment must be approved and documented by the Quality Department.
The following Vendor Management flow chart is the overall vendor/service provider qualification and management process.
Application of (II) Risk Management in Supplier Management
The probability and severity can be rated according to the following model, which also provides the resulting risk.
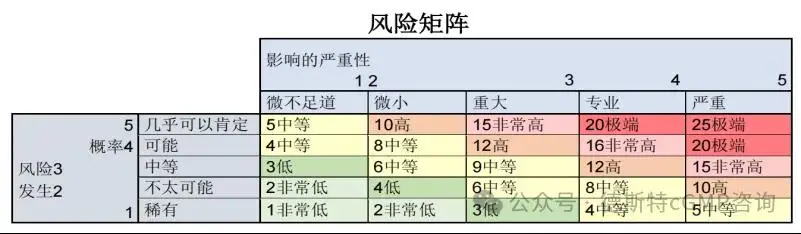
Depending on the size of the calculated risk (from very low to extreme), it is possible to decide what mitigation measures are needed and what priority measures are taken. You can also decide to accept the risk.
If any changes in risk factors are observed during supplier monitoring, the risk assessment must be checked or repeated.
(III) audit
Auditing is one of the most powerful tools for assessing whether a vendor is effectively meeting the needs of an API manufacturer. However, audits of all suppliers of materials and services are not mandatory under GMP.
Audit type: Auditing can be done in different ways.
Ø From a supplier lifecycle perspective => Audit type
o Initial Qualification Review
o Follow-up audit
o Reasons
o Routine audit (monitoring audit)
Ø From the point of view of audit format => audit mode
o Site
o Remote
From the auditor's point of view.
o Own audit
o Third-party audits
o Joint audit
Audit Rating
Typically, the results of an audit can be converted into a classification, for example:
-Acceptable
-Conditional acceptance
-Unacceptable
Reaudit frequency:
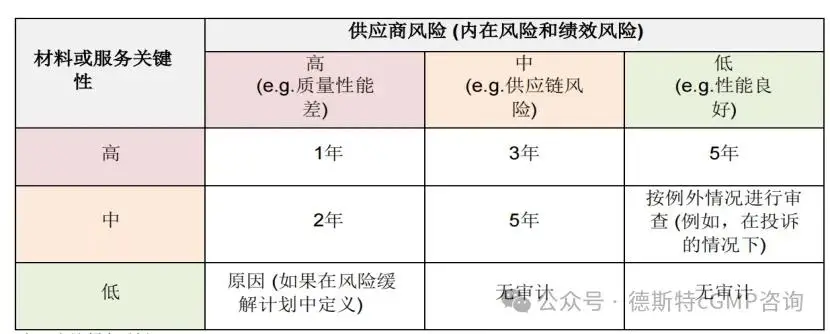
(IV) Quality Agreement
The aim is to define clear (quality) rules and the responsibilities of the parties involved for the materials to be supplied/produced or the services to be supplied. It must be ensured that (quality) requirements are clearly communicated to suppliers and maintained throughout the product life cycle.
If a quality agreement is required due to the risk level of the material or service, it is best to sign it before ordering the material or using the service. If the agreement is (not yet) complete, a risk assessment should be conducted and approval should be obtained from upper management, including at least quality.
In order to be legally binding, a quality agreement should be associated with a supply agreement or by a purchase order. In the absence of a supply agreement, the quality agreement should contain a legal component. If there is a supply agreement, care should be taken not to have conflicting requirements.
Key words:
Related Blog
Durst DST Regulation Group, noting that on July 1, 2024, the Drug Evaluation Center of the State Drug Administration (hereinafter referred to as CDE) issued a trial notice on the submission of electronic application materials for drug registration by network transmission. Starting from July 1, 2024, the trial work on the network transmission of electronic application materials for drug registration will be started, and applicants can submit electronic application materials for drug registration through network transmission. There is also an attachment: the reservation for network transmission of electronic declaration materials and the description of operation steps, which is convenient to guide everyone to submit electronic declaration materials.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Recently, after the products of DST DST customers Sichuan well-known pharmaceutical companies passed PAI inspection in November 2023, they were officially approved in December. In June 2024, they officially sent several batches of products to enter the US market. This exciting news came at the time of the third FDA project audit of the client again. The customer is located in Neijiang City, Sichuan Province, an ancient city between Chengdu and Chongqing. The success of Huiyu Pharmaceutical has made this city more attractive to pharmaceutical people! Once again, congratulations to the customer products officially go to sea! The entry of products into the US market shows that DST customers have been strictly implementing quality management according to high standards, continuously optimizing production processes, and producing more high-quality products higher than national standards.
2024-07-05
Argument! Hengrui Warning Letter, Is It FDA's Political Sanction?
Tieguai Liu saw many posts about whether there were political factors in Hengrui's warning letter. Today, Tieguai Liu will discuss this matter with everyone's concerns. First of all, we are very sensitive to see that the "first brother" of China's pharmaceutical industry has received a warning letter from the FDA of the United States. For today's Sino-US environment, we naturally think of the FDA's sanctions against the first brother due to the influence of US politics. It is completely understandable from everyone's interpretation. The following Tieguai Liu will discuss with you from both positive and negative dimensions:
2024-10-25
Durst DST continues to pay attention to the drug regulatory policies of countries around the world to help companies go to sea, and the following is the drug regulatory situation in Southeast Asian countries and regions.
2024-10-25

