Medical Device GMP Certification
Release time:
2024-10-25
According to the spirit of the national food and drug supervision and administration and the construction of a clean and honest government on December 26, 2013, China will fully implement the production quality management standard in the medical device industry.
Good Manufacturing Practice (GMP) for Medical Devices
The whole process of GMP compliance service is in place in one step.
The Necessity of GMP Certification in Medical Device Factory
According to the spirit of the national food and drug supervision and administration and the construction of a clean and honest government on December 26, 2013, China will fully implement the production quality management standard in the medical device industry. In 2014, the State Food and Drug Administration issued the "Notice on Matters Related to the Implementation of Medical Device Production Quality Management Regulations" (No. 15 of 2014), in accordance with the principles of risk management and classification promotion, it determined different types of medical device manufacturers. The specific time limit requirements for the implementation of the "Regulations. By the end of 2015, all third-class medical device manufacturers in my country must meet the requirements of the "Medical Device Production Quality Management Regulations" (hereinafter referred to as the "Regulations"); since January 1, 2018, all medical device manufacturers must meet the "Medical Device Production Quality Management Regulations" requirements.
The Importance of GMP Certification in Medical Device Factory
Standardize the production process of medical device manufacturers and ensure the standardized operation of products; |
Standardized system to facilitate international integration;
|
Ensure the quality of medical device products, and ensure the safety and effectiveness of products used by the people; |
Improve the technological level of medical device manufacturers and improve the comprehensive competitiveness of medical device products. |

|

|

|

|
Medical Device Factory GMP Certification Service Process
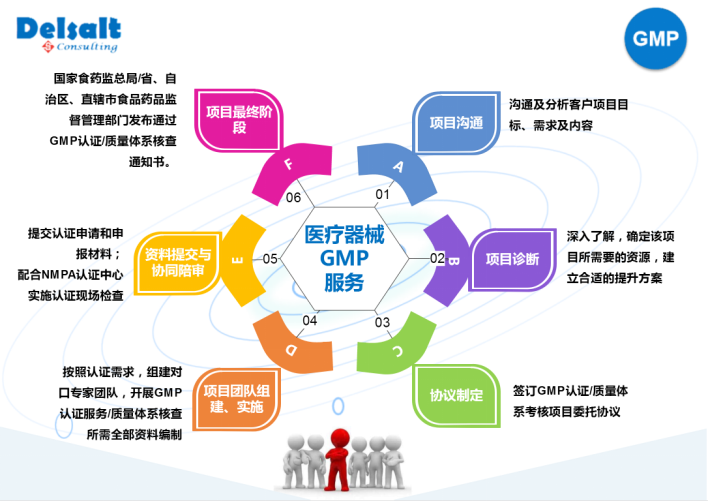
Durst Medical Device GMP Certified Specialist:

5. Durst advantage:
1: the medical device industry regulations, standards of accurate understanding and mastery
2: has a wealth of medical device GMP certification consulting and training experience and on-site technical service experience;
3: professional team, a number of foreign industry experts guidance.
4: one-stop, all-round technical services, etc.
Durst Success Story (partial):

|
Shenzhen Jianke Industrial Co., Ltd. |

|
Yangzijiang Pharmaceutical Group Nanjing Hailing Pharmaceutical Co., Ltd. |

|
Shenzhen Zhonghe Hyderway Biotechnology Co., Ltd. |
For more information, please contact Durst400 9932 368
Previous
Next
Previous:
Next:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

