Drug Marketing Holder System/MAH
Release time:
2024-10-25
"The medical device registrant system issued and implemented by the State Drug Administration has solved the actual dilemma of landing products for science and technology start-up enterprises and technology research institutions, solved the problems of idle production sites, equipment and other resources before listing and unsaturated work of professional manufacturing personnel, and avoided the waste of resources caused by the difference between pre-market trial production and post-market mass production scale, and the occupation of a large amount of infrastructure funds before listing approval.
1. Overview of the Registrant System/MAH
"The medical device registrant system issued and implemented by the State Drug Administration has solved the actual dilemma of landing products for science and technology start-up enterprises and technology research institutions, solved the problems of idle production sites, equipment and other resources before listing and unsaturated work of professional manufacturing personnel, and avoided the waste of resources caused by the difference between pre-market trial production and post-market mass production scale, and the occupation of a large amount of infrastructure funds before listing approval.
2. Registrant System/MAH Service Process
Step one:
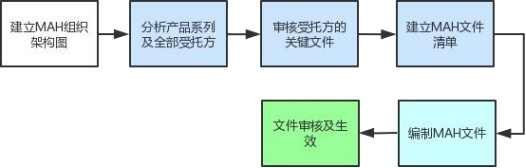
As the MAH holder entrusts a third party to carry out drug production, it is necessary to establish a basic MAH system and system according to the entrustment requirements.
Step two:
According to the requirements of MAH system and regulations, establish corresponding implementation audit for the system. For example, check the standardization of the entrustment contract, establish a quality assurance agreement (involving raw materials, auxiliary materials, packaging materials, etc.), simulate the implementation of recall, etc., and review of deviation changes and other modules.
The management of transportation and sales is also required for sales involving MAH.
Step three:
MAH on-site verification, assist Transocean Medicine to complete MAH on-site verification guidance. On-site assistance to inspection officials
MAH inspection, if necessary, to answer questions, after the inspection is completed, to help MAH check the rectification guidance.
MAH Project Content
Documentation Description File Description |
Item Project |
Note Remarks |
| 1. MAH Organizational Structure | ||
| Combined with the requirements of relevant laws and regulations and guidelines, the MAH management department and the work within the scope of the job responsibilities to analyze and organize the process. | √ |
N/A |
| The relevant management processes of the drug/substance safety committee are analyzed and sorted out. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 2. QA system management | ||
| According to the requirements of relevant regulations and guidelines, analyze and organize the process of each work within the scope of QA responsibilities. | √ |
N/A |
| MAH product release management, document and record management procedures, deviation management procedures, change management procedures, CAPA management procedures, OOS management procedures, internal/external audit, inspection management procedures and risk assessment management procedures are analyzed and sorted out. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 3. Drug Technology Transfer Management | ||
| Combined with the requirements of relevant laws and regulations and guidelines, the technical documents of drugs are analyzed and sorted out. | √ |
N/A |
| Establish detailed technology transfer management system, analysis method transfer, stability management and other document processes, and clarify management requirements and implementation responsibilities. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 4. production plant (trustee) management | ||
| According to the requirements of relevant laws and regulations and guidelines, the process of each work within the scope of responsibility of the trustee shall be analyzed and sorted out. | √ |
N/A |
| Establish the management and control of the corresponding entrustment agreement, quality agreement, release requirements and other processes in the production plant. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 5. supplier management | ||
| Analyze and organize the management process of key material suppliers in accordance with the requirements of relevant regulations and guidelines. | √ |
N/A |
| Establish the corresponding supplier audit system, supplier agreement, etc. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 6. pharmacovigilance workflow analysis and collation, system audit | ||
| Combined with the requirements of relevant laws and regulations and guidelines, the process of each work within the scope of responsibility of the Pharmacovigilance Department is analyzed and sorted out. | √ |
N/A |
| Drug/substance safety committee management procedures, pharmacovigilance protocol management procedures, pharmacovigilance annual report writing and submission management procedures, pharmacovigilance personnel and non-pharmacovigilance personnel training management procedures, safety report management procedures and clinical trial safety information monitoring and serious adverse event reporting management procedures. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
| 7. Drug Sales and Shipment | ||
| Establish requirements related to drug sales and shipment in conjunction with MAH requirements. | √ |
N/A |
| Focus on the delivery system, drug acceptance system, recall and drug transportation management involved in drug sales. | √ |
N/A |
| Final review of revised documents. | √ |
N/A |
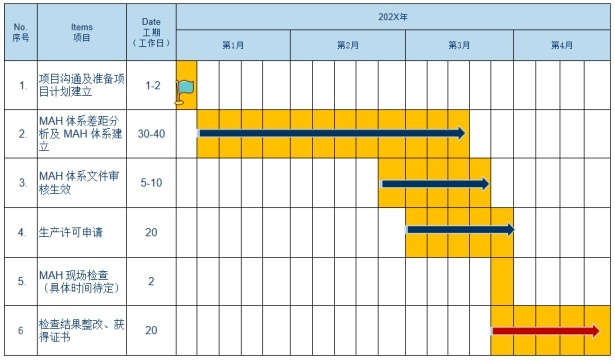
3. MAH Project Implementation Plan
4. Service scope of MAH
(1) Establishment of MAH Regulation Liability System
Provide or help you to establish the organization and personnel system, physical resource system, documents and system of the legal responsibility of the holder.
(2) Material supplier management
1) New original, auxiliary, package supplier selection, due diligence and comprehensive evaluation;
2) the existing original, auxiliary, package suppliers GMP quality system stability supervision and risk warning, GMP daily audit, major change management;
3) Drug quality research and registration changes involved in the addition and change of original, auxiliary and package suppliers.
(3) Contract manufacturer management
1) Contract producer selection, due diligence, comprehensive evaluation;
2) the contract manufacturer GMP quality system stability supervision and risk warning, GMP daily audit, major change management;
3) Drug quality research and registration changes involved in the increase and change of contract manufacturers.
(4) Contract analysis and inspection management
1) Contract laboratory selection, due diligence, comprehensive evaluation;
2) Supervision of the stability of the quality system of the contract laboratory and risk warning, GMP daily audit, data integrity audit, major change management;
3) Change management involved in changing laboratories or adding new laboratories
(5) Sales and contract sales distribution management.
1) GSP quality system stability supervision and risk warning, GSP daily audit.
2) Audit of the warehousing and logistics chain of contract sellers.
3) Management of major changes to contract vendors
(6) Pharmacovigilance and adverse reaction monitoring management
1) Establishment of pharmacovigilance and adverse reaction management system and risk management plan
2) Provision and management of physical resources
3) Adverse drug reaction collection, identification, definition and reporting, adverse reaction review, evaluation, control and adverse signal detection and processing
Periodic Safety Update Report (PSUR)
5) Key drug monitoring and active key monitoring management
6) Response measures management of confirmed serious adverse reactions
(7) Drug registration change and re-registration management
1) Drug change evaluation and classification management
2) Changes and supplementary registration applications due to various reasons
3) Routine re-registration of drugs
Quality System Construction |
Material Supplier Management |
Contract Supplier Management |
Quality system maintenance management |

|

|

|

|
Durst advantage:
1. Have a complete set of problems found, reply to official opinions, formulate rectification measures, guide the implementation of rectification measures, and follow up the effectiveness of the implementation of rectification measures to solve all problems found, and finally successfully pass the audit and official inspection service system.
2, professional team, a number of well-known foreign experts to join the industry, rich audit experience. Has conducted GMP audits for a number of companies.
3. Location advantage. Durst has offices in East China, South China and Southwest China, which is more convenient for face-to-face communication and inspection with enterprises.
4, one-stop, all-round technical services, etc.
Durst Success Story (partial):
Overseas Medicine (Guangzhou), China Resources Sanjiu Medicine, Zhuhai Lizhu Monoclonal Biology, Zaiding Medicine (Suzhou) Co., Ltd., Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd., Shenzhen Zhonghe Hedway and other MAH projects have successfully passed the inspection.
Zhangzhou Pien Tze Huang Pharmaceutical Shares: MAH Holder (Add XX Oral Solution, XX and other 2 varieties of production address, production scope and other information). At present, the MAH project of the first entrusted site has been completed.
The latest completion of the drug production license (B license) project: March 29, 2021, to help overseas medicine to obtain the drug production license.
In 2021, Transocean Pharmaceutical (Guangzhou) Co., Ltd., as the holder of MAH, has successfully obtained the pharmaceutical production license (B certificate) for chemical drugs (XX).
In October 2022, Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd., production site: Shandong Lonuo Pharmaceutical.
In July 2023, help Beijing Aoxin Sunshine (Beijing Haiyi) complete MAH project, successfully pass and obtain B certificate;
In September 2023, he helped Ping An Yaneyi (a Sino-Japanese joint venture) complete the MAH project, successfully passed and obtained the B certificate.

As one of the publicity and implementation units of the Drug Administration Law, Durst has participated in the training of drug administration law, including MAH regulations, and has certain advantages in the implementation of MAH regulations.

Previous
Previous:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

