PIC/S-GMP Certification
Release time:
2024-10-25
PIC/S GMP certification is an internationally recognized pharmaceutical production quality management practice certification, which aims to ensure the consistency of pharmaceutical production and quality control standards in participating countries, thereby promoting international pharmaceutical trade. Obtaining PIC/S GMP certification means that pharmaceutical companies meet international standards in drug production and quality management, thereby increasing the competitiveness of drugs in the international market. Pharmaceutical companies want to sell drugs in the international market, and obtaining PIC/S GMP certification is a key step.
Introduction of 1. PIC/S:

PIC/S GMP certification is an internationally recognized pharmaceutical production quality management practice certification, which aims to ensure the consistency of pharmaceutical production and quality control standards in participating countries, thereby promoting international pharmaceutical trade. Obtaining PIC/S GMP certification means that pharmaceutical companies meet international standards in drug production and quality management, thereby increasing the competitiveness of drugs in the international market. Pharmaceutical companies want to sell drugs in the international market, and obtaining PIC/S GMP certification is a key step.

The Pharmaceutical Inspection Co-operation Scheme (PIC/S) was established in November 1995. It is the only international cooperation organization in the world composed of GMP inspection authorities of various countries. Its purpose is to eliminate obstacles in drug trade, improve the consistency of drug licensing, ensure drug quality, promote the harmonization of international GMP regulations and standards and the consistency of GMP inspection quality.
PIC/S now has 50 countries or regions, such as Australia, Austria, Belgium, Canada, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Malaysia, the Netherlands, Norway, Poland, Portugal, Romania, Singapore, Taiwan, Hong Kong, South Korea, Slovakia, Spain, Sweden, Switzerland and the United Kingdom, etc. The organization enjoys a high reputation in the world, and its internal prosecutors are all from relevant professional authorities in each member country, and they are also representatives of participating official (regulatory agencies). The member states of the organization have consistent GMP specifications and inspection systems, and mutually recognize the inspection results. The GMP certificates issued by the organization are mutually recognized among the member states of the PIC/S organization. Passing the PIC/S-GMP certification of a certain member state also means stepping into the first threshold of 50 countries or regions. Therefore, it is one of the fast channels to enter the international market.
2. PIC/S GMP Certification Service
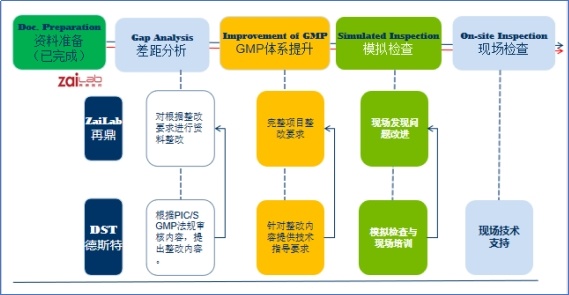
1. Internal preparation and evaluation
DST DST helps pharmaceutical companies to conduct a comprehensive assessment of their own production and quality management systems to ensure that their quality and production meet PIC/S GMP requirements. This may require upgrading or modification of production facilities, equipment, procedures, personnel training and quality control systems.
2. Establish quality management system
Pharmaceutical companies need to establish and improve the quality management system, including quality control, quality assurance, verification, change management, complaint handling, product recall and other aspects. DST can help enterprises to build and improve the quality management system.
3. Training employees
DST conducts PIC/S GMP training for customer employees to ensure that they understand and can comply with GMP requirements.
4. Pre-inspection and internal audit services
Before formally applying for PIC/S GMP certification, DST DST can conduct pre-inspection or internal audit for pharmaceutical companies to identify and solve possible problems and lay a good foundation for formal audit.
7. Assist in certification
Assist enterprises to organize GMP approval materials and submit GMP certification applications to the drug regulatory agencies of PIC/S member countries or regions through the drug regulatory agencies of the country where they are located.
8. Assist official inspection
Provide on-site technical and technical translation support during on-site GMP compliance inspection for PIC/S certification;
9. Assist in rectification and feedback
If the inspection process found does not meet the requirements of PIC/S GMP, to assist the pharmaceutical companies to complete the rectification.
PIC/S GMP Certification Case
Durst has helped many enterprises to carry out the overall implementation of PIC/S projects. The PIC/S projects involved include: Malaysia project, Philippines project, Singapore project, Hong Kong project, Taiwan project, Eastern European project, Brazil project, Eastern European country project, etc. Some enterprises have relatively weak foundation, but they have successfully completed PIC/S project after systematic rectification.
1. TGA certification of a state-owned enterprise in Shenzhen

2. Singapore PIC/S Project of a Pharmaceutical Company in Nanjing

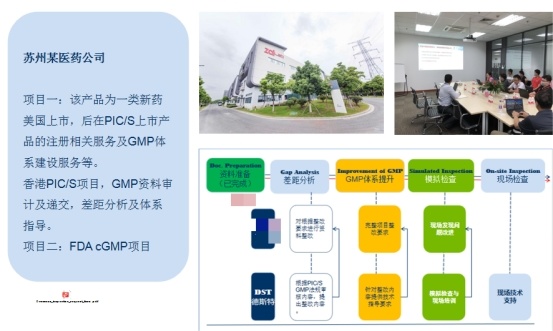
3. Hong Kong PIC/S Certification Project of a Pharmaceutical Company in Suzhou
4. A biomedical company in Shenzhen

5. For the PIC/S project of a well-known central enterprise in Shenzhen, 4 workshops on site passed PIC/S inspection at the same time.

Wait.....
Previous
Previous:
Next:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

