FDA cGMP Certification
Release time:
2024-10-25
FDA(Food and Drug Administration) is the food and drug regulatory agency authorized by the United States Congress, which is responsible for supervising and regulating food, drugs, medical devices, cosmetics and other products related to public health. The FDA's primary mission is to ensure the safety, effectiveness, and proper labeling of these products when used by the public.
1. FDA GMP Certification
FDA(Food and Drug Administration)It is a food and drug regulatory agency authorized by the U.S. Congress to supervise and regulate food, drugs, medical devices, cosmetics, and other public health-related products. The FDA's primary mission is to ensure the safety, effectiveness, and proper labeling of these products when used by the public.

In common usage, "FDA certified" is not an official term used by the FDA. People often use "FDA certification" to refer to the following three situations:
FDA Registration:For food, drug and medical device companies exporting to the United States, they must register with the FDA, including the listing of the company and products. If the registration is not completed, the customs will not be cleared. This is a mandatory requirement;
FDA Testing:FDA testing usually refers to the safety testing of food contact materials, product packaging materials, biocompatibility testing and clinical safety testing of medical products. These tests are designed to ensure product safety and compliance with FDA standards;
FDA approved:FDA approvals are primarily in the area of pharmaceuticals. When a drug has undergone rigorous research, testing, and evaluation, it is approved by the FDA before it can be sold and used in the U.S. market.
2. Project Objectives
Durst DST helps companies to pass the US FDA certification within the time agreed with the company, and helps companies to improve the legal procedures for their products to enter the US and European markets, so that their products can enter the US market smoothly. The legal listing of Chinese domestic products in the European and American markets will have a positive impact on the products in the domestic market and other international markets.
3. service mode
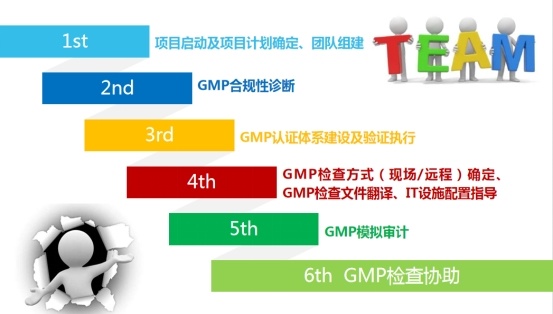
1. Gap assessment of the existing quality management system and comparison with FDA regulations;
2. Overall design, understand the existing company structure, production system, QA system, QC system, supply chain system, engineering system, etc;
3. FDA training, the company's relevant responsible personnel and employees for FDA drug general regulations training;
4. Special training on FDA regulations, special training on FDA drug regulations for relevant persons in charge of the company;
5. Collect the company's existing quality documents and improve the quality document system;
6. Implementation of quality documentation system;
7. Check the effectiveness of the system;
8. Check the effectiveness of the system, before the FDA to audit, arrange the company's reviewers to conduct a simulation audit;
9. Accompany FDA to review the factory and translate;
10. Assist enterprises to rectify the non-conformances proposed by FDA review plants until FDA closes the non-conformances.
4. FDA factory inspection experience sharing:
The greater the amount of production and export to the United States, the more users complain, and the greater the possibility of being inspected by FDA;
2. However, enterprises in China (including Hong Kong, Macao and Taiwan), whether they are Class I, Class II or Class III, have a very high probability of being inspected by FDA, and our company has come into contact with a large number of cases of low-risk product inspection;
3. All inspection fees, air tickets, travel, five-star hotels, meals and other expenses are borne by FDA;
FDA usually notifies the factory 1-3 months in advance, but will not notify the specific date of the factory;
5. Usually FDA only comes to one person, normal audit 4 days;
6. Important documents such as quality manuals and procedure documents shall be translated into English, and environmental sanitation shall be well done;
7. FDA more emphasis on internal audit and records, the relevant departments of the signature;
8. FDA attaches great importance to laws and regulations, professional knowledge, operating guidelines and other staff training and implementation, signature;
9. If there is any non-conformity, the auditor will issue a 483 form on site;
10. For all questions raised by the auditor, the enterprise must submit a written reply within the specified time, the sooner the better;
11. For major non-conformations, the auditor will issue a warning letter (Warning letter) on the spot, and the enterprise must complete the rectification within the specified time.
4. FDA Case
1. FDA Project of a Pharmaceutical Enterprise in Zhejiang

2. FDA Project of a Pharmaceutical Enterprise in Guangdong
3. Injection FDA Project of a Pharmaceutical Company in Sichuan

4. FDA Project of Raw Material Drug of a Biological Enterprise in Chengdu
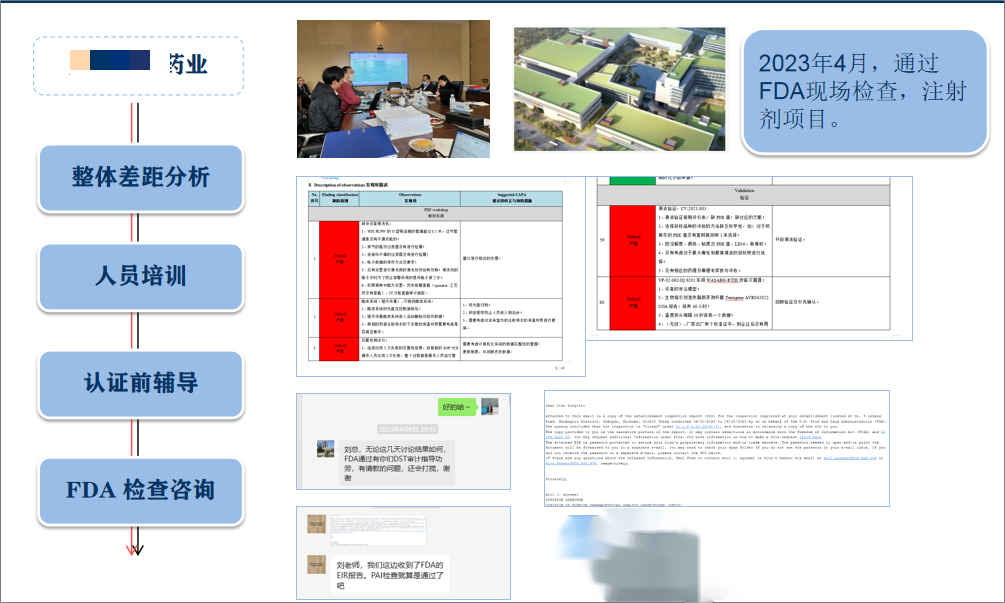
5. FDA Project of Sterile Preparations of a Pharmaceutical Enterprise in Hubei Province

6. OTC FDA Project of a Famous Central Enterprise in Shenzhen

7. FDA Project of a Pharmaceutical Enterprise in Fujian

8. API FDA Project of a Pharmaceutical Company in Jiangsu Province

9. Other FDA Program Customers

If you need FDA certification related services, please contact 18826007039/13326993435 (WeChat with the same number) ~
Previous
Previous:
Next:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

