EU GMP Certification
Release time:
2024-10-25
EU GMP certification is a mandatory manufacturing standard for drugs and medical devices sold on the market of EU member countries. The European Union (EU) is one of the world's largest and most important international mainstream markets for pharmaceuticals. Since the GMP certification and inspection results of nearly 30 EU member states are mutually recognized, the EU and the United States, Japan, Australia and other countries have gradually begun to recognize each other's GMP certification.
1. EU GMP Certification
EU GMP CertificationIt is a mandatory manufacturing standard for drugs and medical devices sold on the market of EU member countries. The European Union (EU) is one of the world's largest and most important international mainstream markets for pharmaceuticals. Since the GMP certification and inspection results of nearly 30 EU member states are mutually recognized, the EU and the United States, Japan, Australia and other countries have gradually begun to recognize each other's GMP certification.

After obtaining the EU GMP certification, the product can enter the large market of nearly 0.6 billion people in the EU. At the same time, it can also improve the management level of enterprises and obtain more domestic preferential policies.
2. how to pass the EU GMP certification
Due to the implementation of China's new version of GMP (version 2010), the hardware equipment and facilities of Chinese pharmaceutical companies have gradually been in line with international equipment and facilities. Companies only need to transform on the existing basis, and it will be close to the international market. far away.
The main procedures for applying for an official inspection are:
1, to become the EU pharmaceutical companies commissioned processors
2. Apply for drug marketing authorization in the EU
3, through the EU importers to export products to the EU
Certification Procedure
In principle, European GMP certification is related to "drug marketing". The EU or its member states do not have an independent GMP certification/inspection procedure. To apply for EU GMP certification, there must be a procedure to "start (Trigger)".
(I) for pharmaceutical manufacturers, GMP certification inspection "start-up" procedures are:
1, the European Union "Drug Marketing Authorisation Application (MAA)";
Importing medicines into the EU through EU importers;
3, accept the EU pharmaceutical company "contract/custom production";
4. Become an overseas production plant for EU pharmaceutical companies.
(II) for raw material drug manufacturers, the "start-up" procedure mainly includes:
1, "European Pharmacopoeia Applicability Certificate (CEP/COS)" application;
2. Become a supplier of raw materials for pharmaceutical preparations listed in the EU;
3. EU GMP Certification Services
DST DST has been engaged in GMP certification consulting and counseling in Europe and the United States for many years, helping European and American pharmaceutical companies and domestic pharmaceutical companies to pass the EU GMP certification inspection for many times. To help more pharmaceutical companies go international, the following is our service
Process:
On-site Audit and Differentiation Analysis of (I) Enterprises
1. Investigate the current GMP level implementation status of pharmaceutical companies, audit the factory, and confirm the gap with the EU GMP level;
2. According to the on-site audit, formulate the audit report;
3. Formulate corresponding project rectification suggestions according to the audit report;
4. Communicate with customers to form the final project construction strategy;
5. Combined with the actual situation of the enterprise, put forward the overall opinion, discuss and determine the policies and strategies for the project.
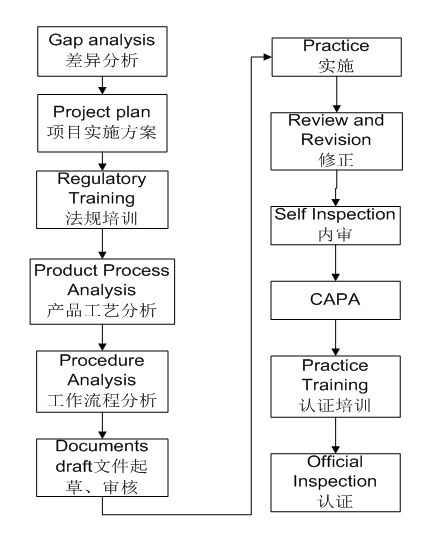
Technology Transfer of (II) Products and EU GMP Certification
1. Assist companies to select suitable products and identify countries that require GMP certification;
2. Assist enterprises to sign product transfer or commissioned production agreements;
3. Cooperate with pharmaceutical companies to complete product registration;
4. Help enterprises to complete product technology transfer;
5. Assist enterprises to complete the construction of GMP system and verification system;
6.GMP approval data collation and submission;
7. Provide experts, consultants and technical personnel to conduct joint "simulation inspection" of the enterprise ";
8. Provide on-site technical and technical translation support during on-site GMP compliance inspections of production facilities by EU or Member State officials;
9. Guide and assist enterprises in the correction and prevention of inspection defects (CAPA) and feedback to European inspectors.
10. EU listing approval and commercial production of enterprise products.

5. EU GMP certification case
Durst DST team has implemented dozens of European and American GMP projects, and has been recognized by customers, involving EU GMP solid products, non-final sterilization injection products, final sterilization injection products, raw materials and other different types of products.

If you need FDA certification related services, please contact 18826007039/13326993435 (WeChat with the same number)
Previous
Previous:
Next:
Related Content
Plant design engineering construction management
Combined with the rich experience of the project team, the verification system and GMP system are optimized and improved according to the existing configuration and operation of the enterprise. Durst advocates the introduction of new verification ideas and concepts in Europe and the United States to promote project implementation:
2024-10-25
The formation of the quality of any drug is designed and produced, not tested. How to ensure that the quality of the product remains stable and high quality?
2024-10-25
Products registered in Europe and America
Durst (Delsalt) is a global pharmaceutical consulting company, maintaining exchanges and contacts with multiple industry associations in Europe and the United States. We have a wealth of global drug information resources, including solid oral preparations, injections, eye drops, and topical products. Durst has rich experience in the registration of products in developed countries such as the European Union and the United States, and has helped many pharmaceutical companies to complete the registration of products in the European Union and the United States. According to the needs of pharmaceutical companies, to help pharmaceutical companies through different ways to enter the developed European and American markets.
2024-10-25
European and American drug OEM
As a Spanish global consulting company, Delsalt has a wealth of product resources in Europe and the United States to meet the OEM needs of major customers in the current market. Entrusted processing (Original Equipment Manufacturer,OEM)& contract processing outsourcing (Contract Manufacture Organization,CMO): European drug listing license holding companies, entrusted to Chinese pharmaceutical enterprises production, entrusted companies to assist Chinese pharmaceutical enterprises to obtain EU GMP certification, production of drugs sold to the entrusted company, by the entrusted company sold to EU member states.
2024-10-25
New Drug Transfer in Europe and America
In countries such as Europe, the United States, Japan or India, their drug research and development technology is becoming more and more mature, and their research and development institutions have developed new drugs that are in the clinical stage or on the market. Through its resources in Europe and the United States, Delsalt can help companies obtain new drug transfer information from R & D institutions in the United States, Europe and Japan.
2024-10-25
QP Declaration of Conformity is a formal declaration of GMP conformity in the clinical phase of the product in the EU and PIC/S Member States, which has the effect of legal recognition, and the inspection in the clinical phase is equivalent to the official inspection. The QP shall be responsible for the GMP implementation results of the enterprise. In the clinical phase, the QP conformity statement can be submitted as GMP conformity data in the clinical phase to the EU or PIC/S countries as the basis for GMP conformity. The QP declaration of conformity is also a very good proof of compliance before the enterprise has not obtained the official GMP certificate overseas. According to the EU QP regulation, except in special circumstances, the QP declaration of conformity needs to be checked remotely, the enterprise or local government provides proof, and the QP should go to the site for review.
2024-10-25

